Boyle S And Charles Law Worksheet
Boyle S And Charles Law Worksheet - We are going to study 2 of the famous gas laws: What is the equation for boyle’s law?_____ 2. What does the letter p stand for? What is the new volume? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. Boyle’s law worksheet fill in the blanks. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional.
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume? Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. What is the equation for boyle’s law?_____ 2. We are going to study 2 of the famous gas laws: When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law worksheet fill in the blanks. What does the letter p stand for? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.
Boyle’s law worksheet fill in the blanks. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the equation for boyle’s law?_____ 2. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. We are going to study 2 of the famous gas laws: Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. What does the letter p stand for? What is the new volume?
Gas Laws Presentation Chemistry
What is the new volume? Boyle’s law worksheet fill in the blanks. What does the letter p stand for? Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. We are going to study 2 of the famous gas laws:
(DOC) Gas laws worksheet 2 boyles charles and combined (1)
What is the new volume? Boyle’s law worksheet fill in the blanks. We are going to study 2 of the famous gas laws: Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional.
Boyle's Law Questions And Answers
Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. We are going to study 2 of the famous gas laws: When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What does the letter p stand for? When ____________ is held constant, the pressure and volume of a gas.
The Theories and Behavior of Gas Owlcation
We are going to study 2 of the famous gas laws: When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law worksheet fill in the blanks. Boyle’s law, which looks at the relationship between pressure and.
What Does Boyle's Law Describe
When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. We are going to study 2 of the famous gas laws: A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s..
Worksheet Boyle's Law And Charles Law
What is the equation for boyle’s law?_____ 2. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the new volume? Boyle’s law worksheet fill in the blanks.
Charle’s vs Boyle’s Gas Laws anchor chart Teaching chemistry
What is the equation for boyle’s law?_____ 2. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. What is the new volume? Boyle’s law worksheet fill in the blanks.
Gas Laws Worksheet 2 Boyles Charles and Combined Gases Pressure
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the equation for boyle’s law?_____ 2. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. When ____________ is held constant, the pressure and volume of a gas are ____________.
Charles And Boyles Law Worksheet
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Boyle’s law worksheet fill in the blanks. What is the new volume? What does the letter p stand for? We are going to study 2 of the famous gas laws:
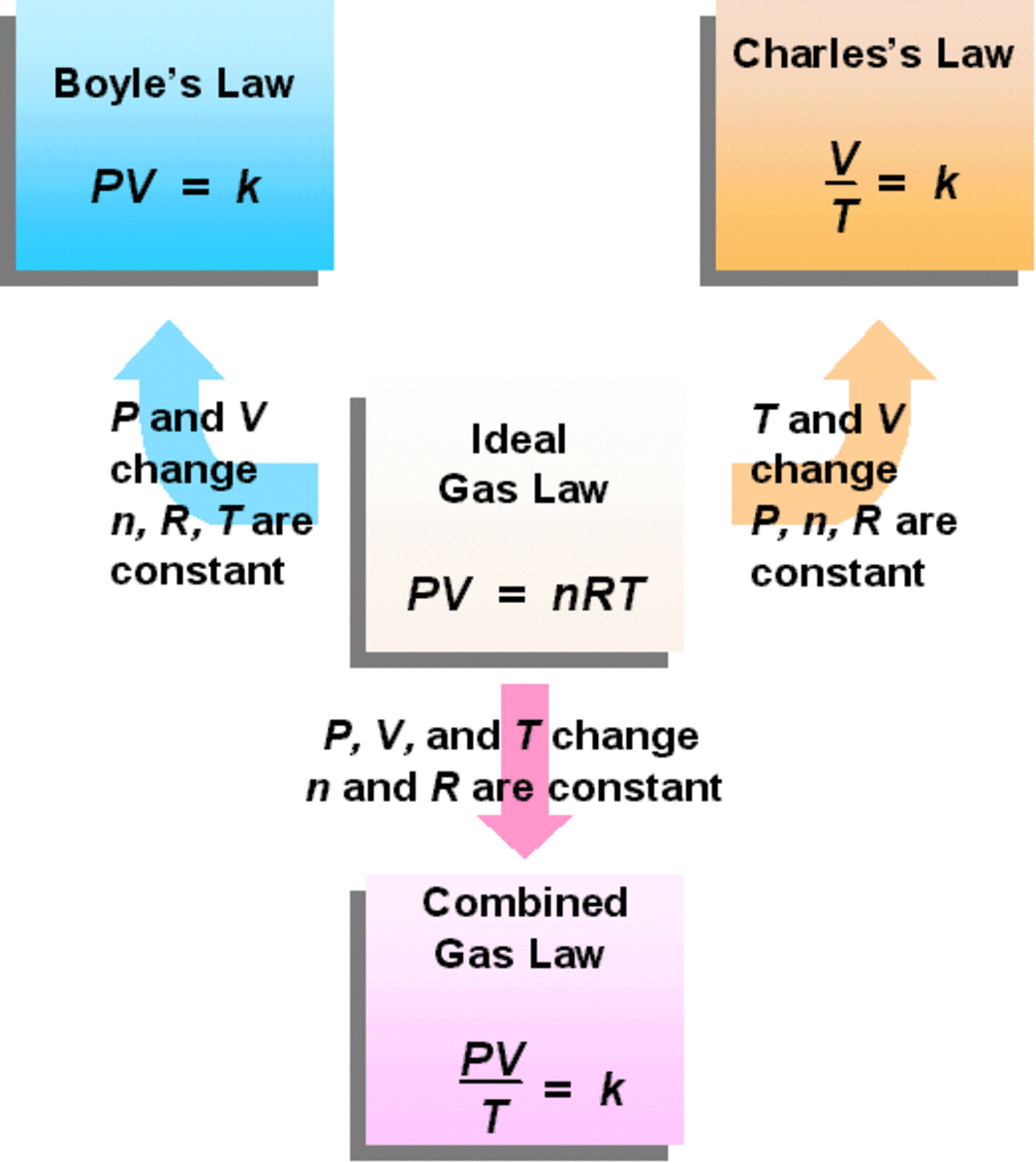
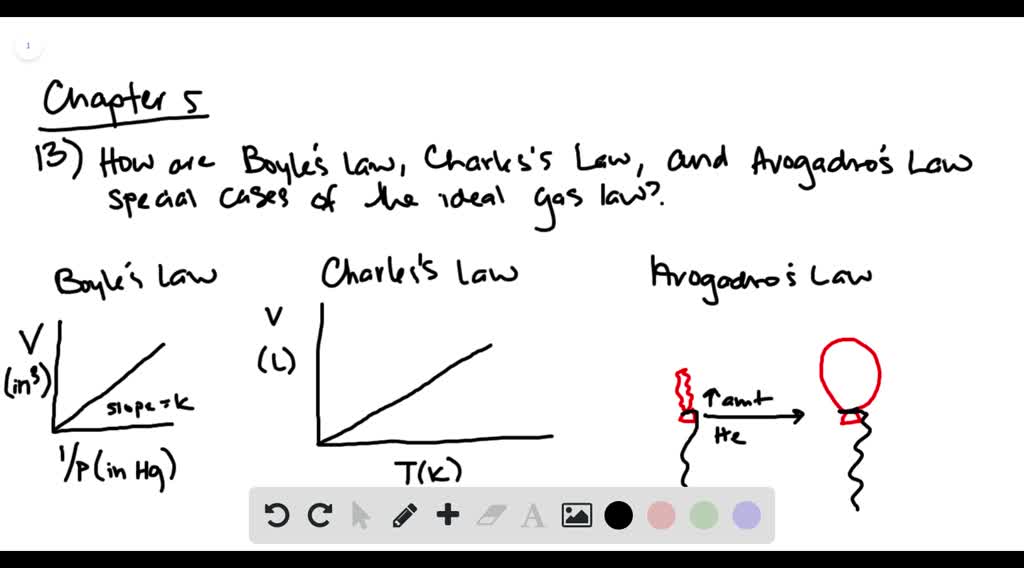
SOLVED Explain how Boyle’s law, Charles’s law, and Avogadro’s law are
When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law worksheet fill in the blanks. What is the equation for boyle’s law?_____ 2. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.
When ____________ Temperature Is Held Constant, The Pressure And Volume Of A Gas Are ____________ Inversely Proportional.
Boyle’s law worksheet fill in the blanks. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume? When ____________ is held constant, the pressure and volume of a gas are ____________ proportional.
We Are Going To Study 2 Of The Famous Gas Laws:
Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. What does the letter p stand for? What is the equation for boyle’s law?_____ 2.
.PNG)