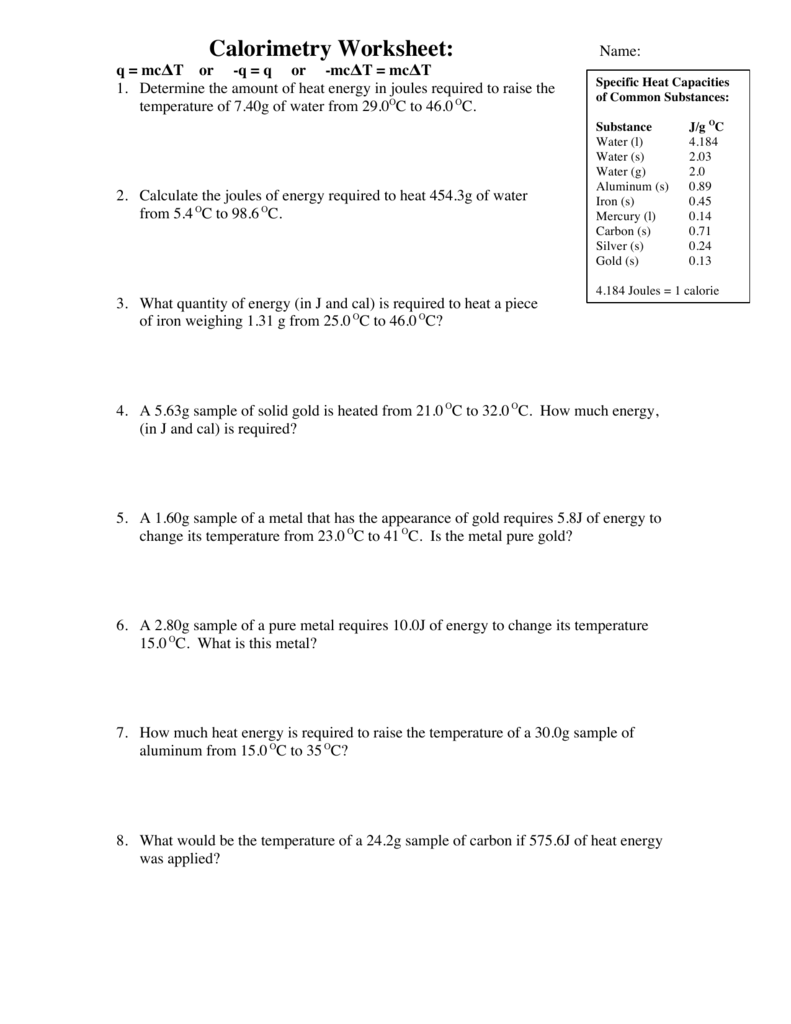
Calorimetry Problems Worksheet Answers
Calorimetry Problems Worksheet Answers - Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry questions and problems 1. If the combustion of 0.285 moles of this compound causes the. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively.
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. If the combustion of 0.285 moles of this compound causes the. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the.
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this compound causes the. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively.
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. If.
Calorimetry Problems Worksheet With Answers
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Problem \(\pageindex{8}\) when 1.0.
Calorimetry Worksheet Answers Pogil Printable Word Searches
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits,.
Chemistry Calorimetry Problems 1 Answers
The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. If the combustion of 0.285 moles of this compound causes the..
Calorimetry Worksheet 1 Answers Heat Capacity Calorimetry Worksheet
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. If the combustion of 0.285 moles of this compound causes the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l.
Calorimetry Questions And Answers
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry.
Calorimetry Practice Problems With Answers Calorimetry Pract
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this compound causes the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry worksheet 1) if.
SOLUTION Phys1020 unit 5 worksheet 1 specific heat calorimetry Studypool
Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned.
50 Calorimetry Worksheet Answer Key
If the combustion of 0.285 moles of this compound causes the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry questions and problems 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes.
Calorimetry Worksheet with Answer Key Exercises Chemistry Docsity
Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h.
The Specific Heats Of Water And Iron Are 4.184 J/Goc And 0.44 J/Goc Respectively.
Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. If the combustion of 0.285 moles of this compound causes the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
Calorimetry Questions And Problems 1.
Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water.