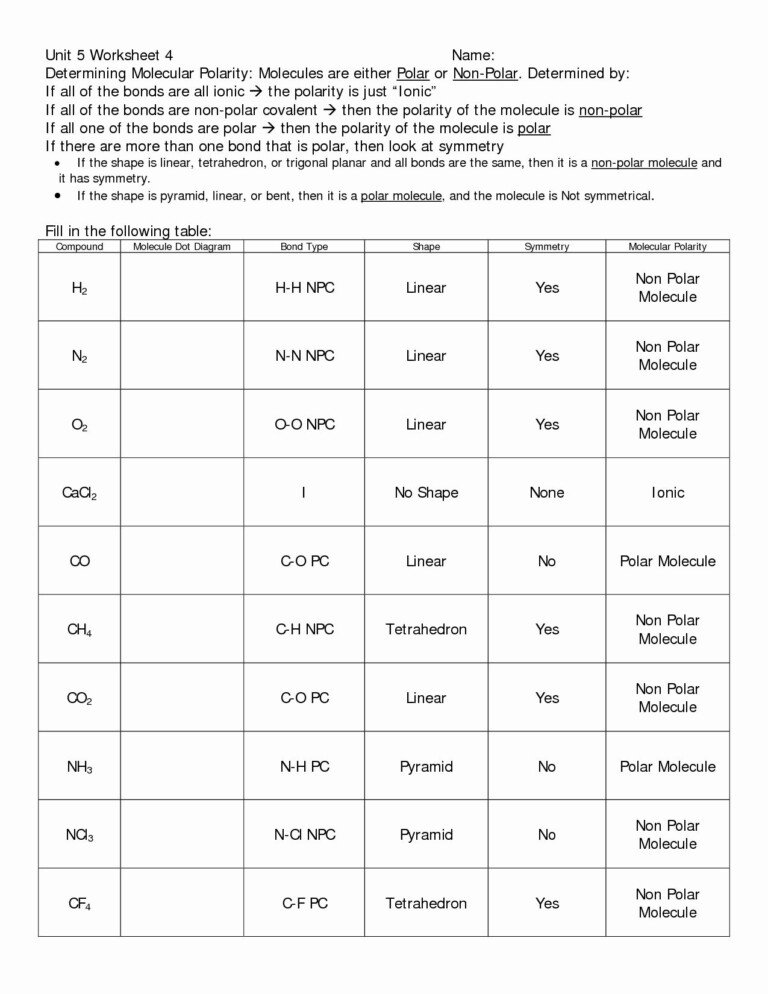
Covalent Bonding Practice Worksheet
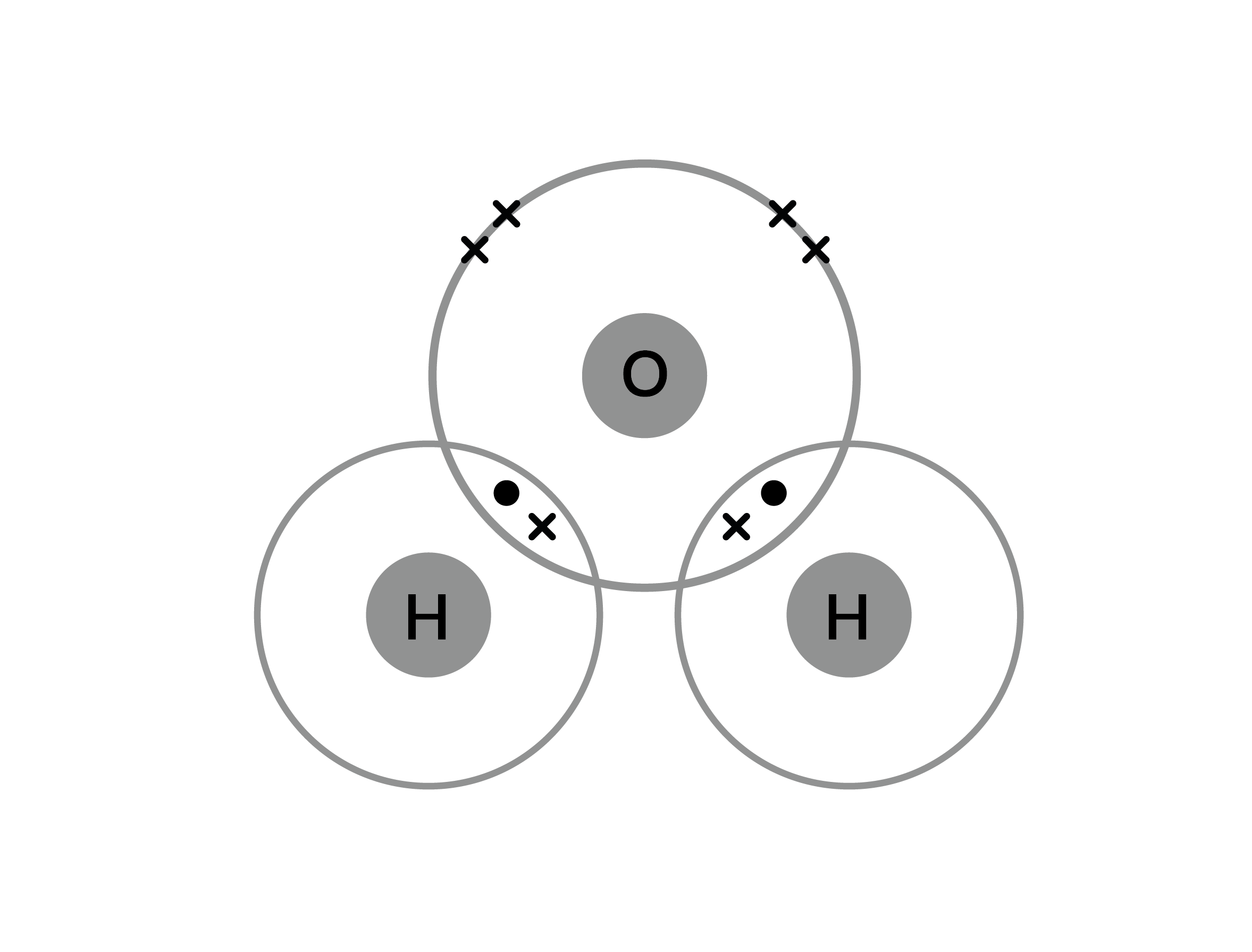
Covalent Bonding Practice Worksheet - Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow.
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow.
Covalent bonding Chemistry Explanation & Exercises evulpo
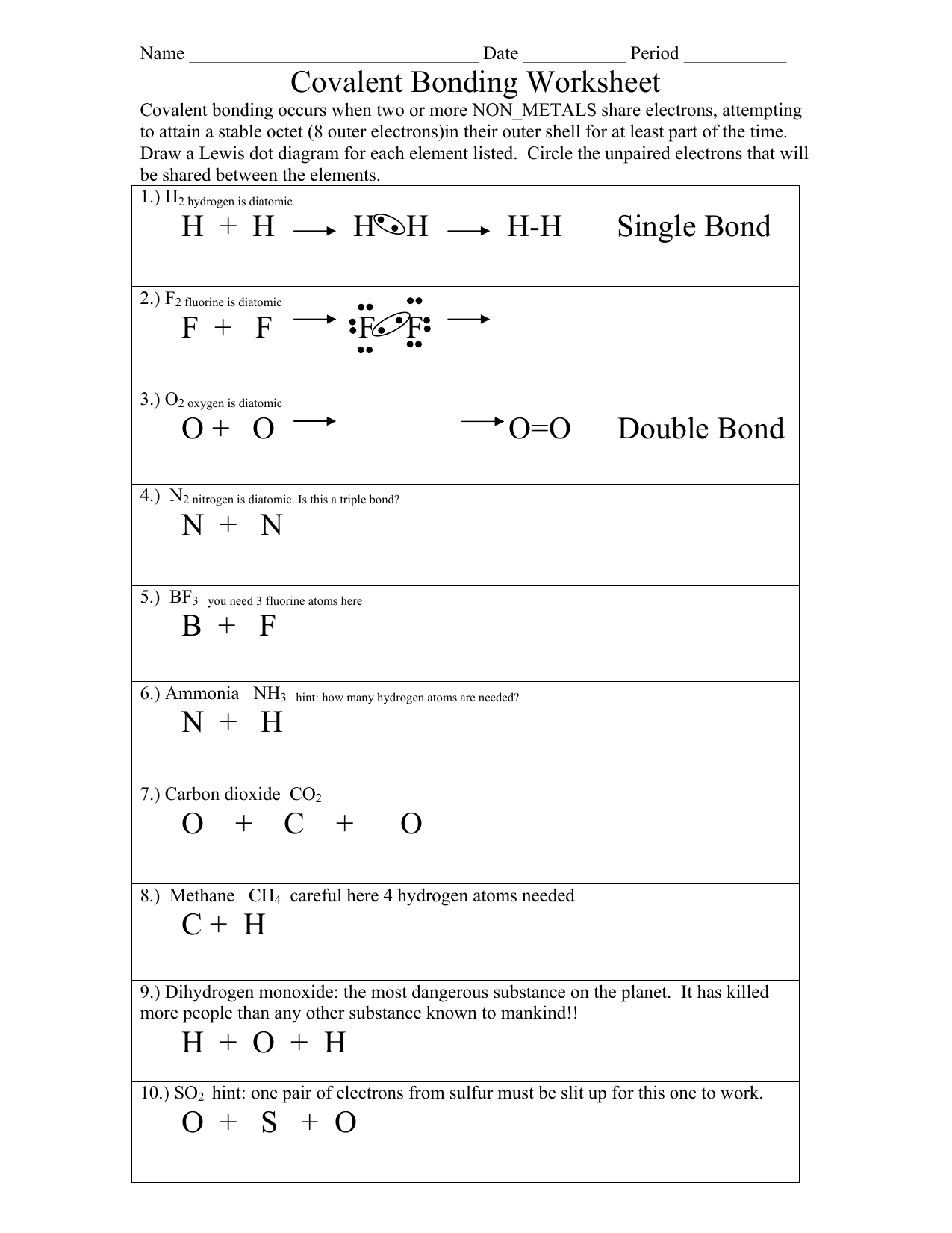
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Practice Drawing Covalent Bonds
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow.
Covalent Bonding Worksheet
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Ionic And Covalent Bonding Practice Worksheet
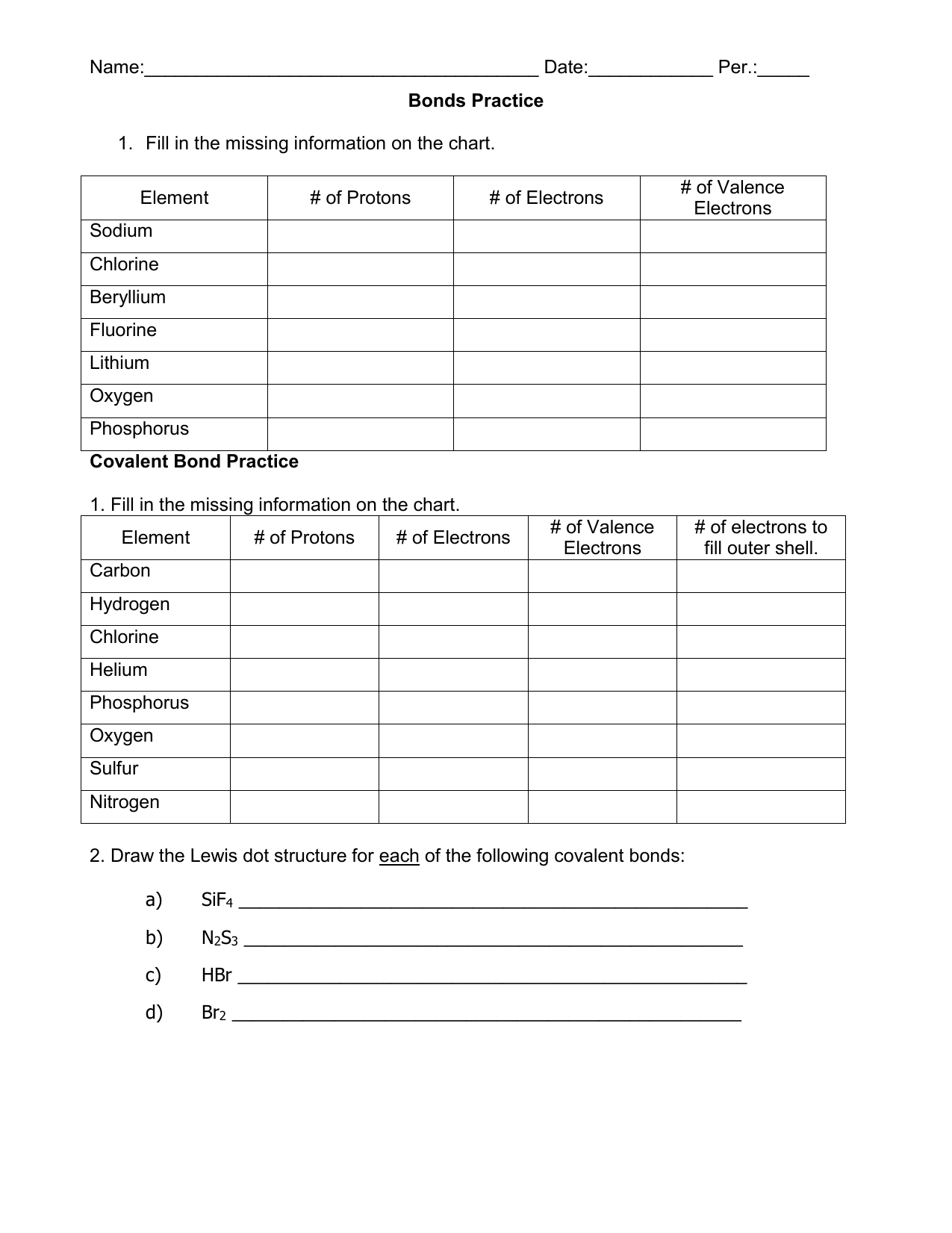
Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Free Printable Covalent Bonding Worksheets Worksheets Library
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow.
Covalent Bonding Naming Worksheet
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow.
Quiz Covalent Bonds
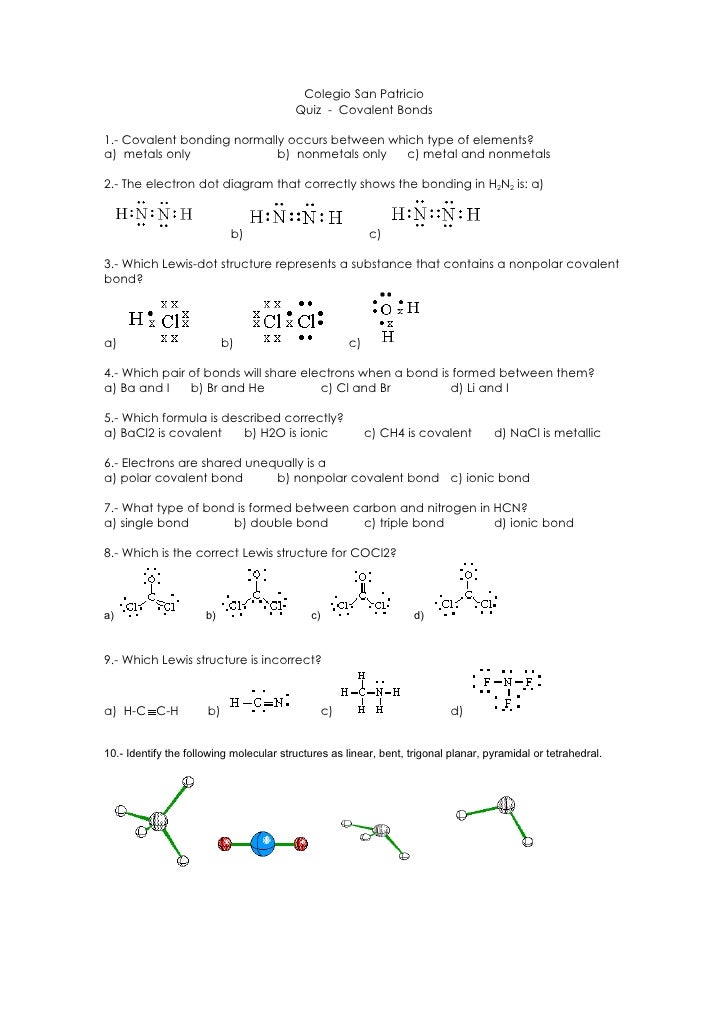
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Quiz & Worksheet Covalent Chemical Bonds
Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Ionic And Covalent Bonding Practice Worksheet Worksheet
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Naming Ionic And Covalent Bonds Practice
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Write A Simple Rule That Will Allow.
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.