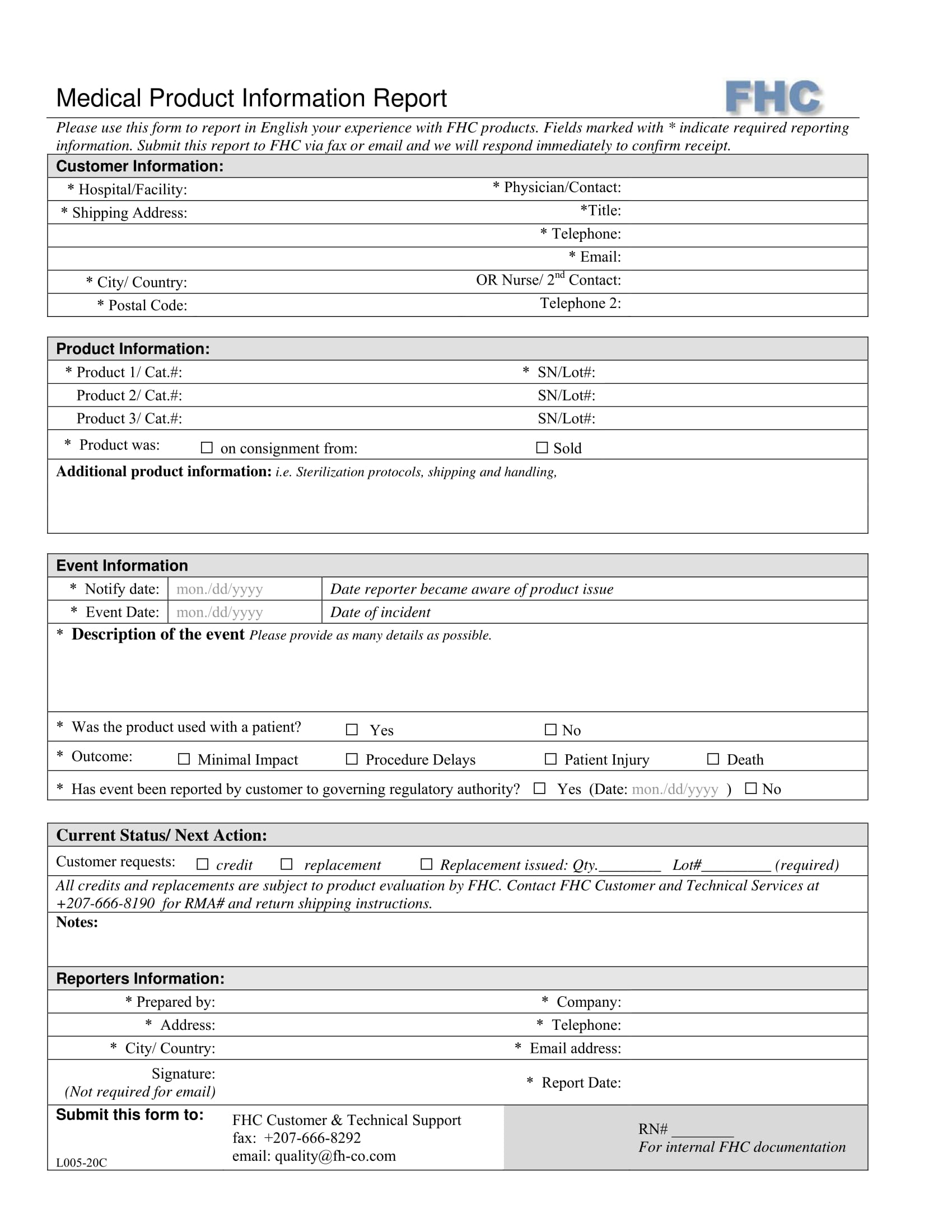
Ema Product Information Templates
Ema Product Information Templates - European medicines agency website (e.g. It presents the electronic product information (epi) process that will be used during the pilot. This guide is for applicants participating in the epi pilot. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical.
The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. This guide is for applicants participating in the epi pilot. It presents the electronic product information (epi) process that will be used during the pilot. European medicines agency website (e.g. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for.
European medicines agency website (e.g. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. This guide is for applicants participating in the epi pilot. It presents the electronic product information (epi) process that will be used during the pilot.
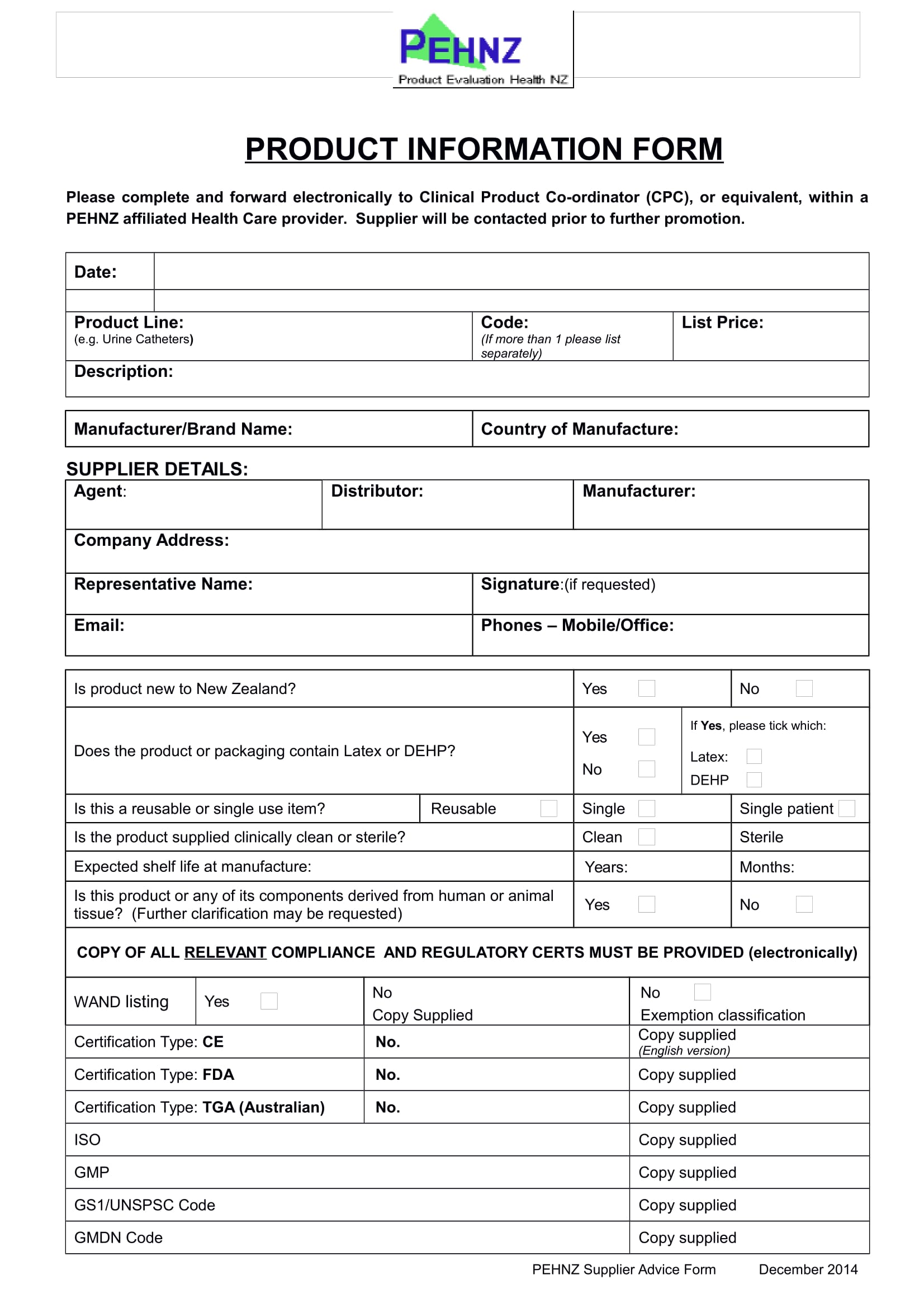
FREE 14+ Product Information Forms in MS Word PDF Excel
It presents the electronic product information (epi) process that will be used during the pilot. This guide is for applicants participating in the epi pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. European medicines agency website (e.g. The european medicines agency's (ema) working group on quality review of documents (qrd).
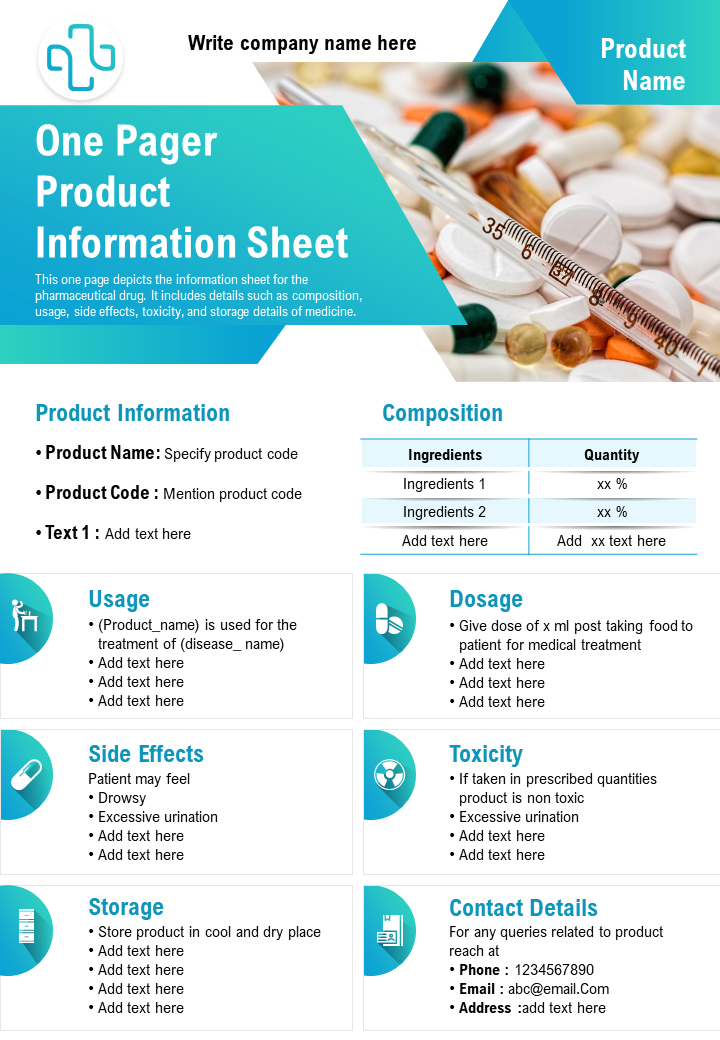
Top 10 OnePage New Product Fact Sheet Templates The SlideTeam Blog
European medicines agency website (e.g. It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. This guide is for applicants participating in the epi pilot. The european medicines agency (ema) makes guidance and templates available to.
Ema Product Information Templates
The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. This guide is for applicants participating in the epi pilot. European medicines agency website (e.g. It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to.
Ema Product Information Templates
It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. This guide is for applicants participating in the epi.
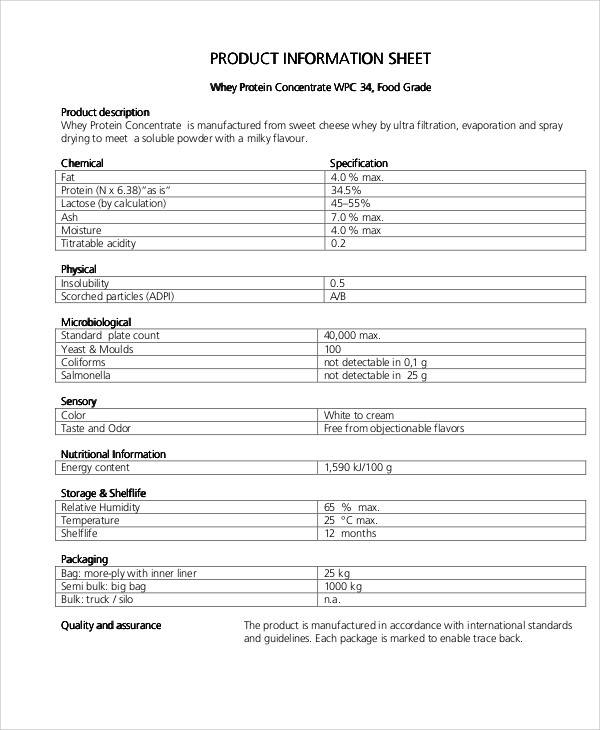
Product Data Sheet Templates Free Printable Templates
It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. European medicines agency website (e.g. This guide is for.
Ema Product Information Templates
European medicines agency website (e.g. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. This guide is for applicants participating in the epi pilot. It presents the electronic product information (epi) process that.
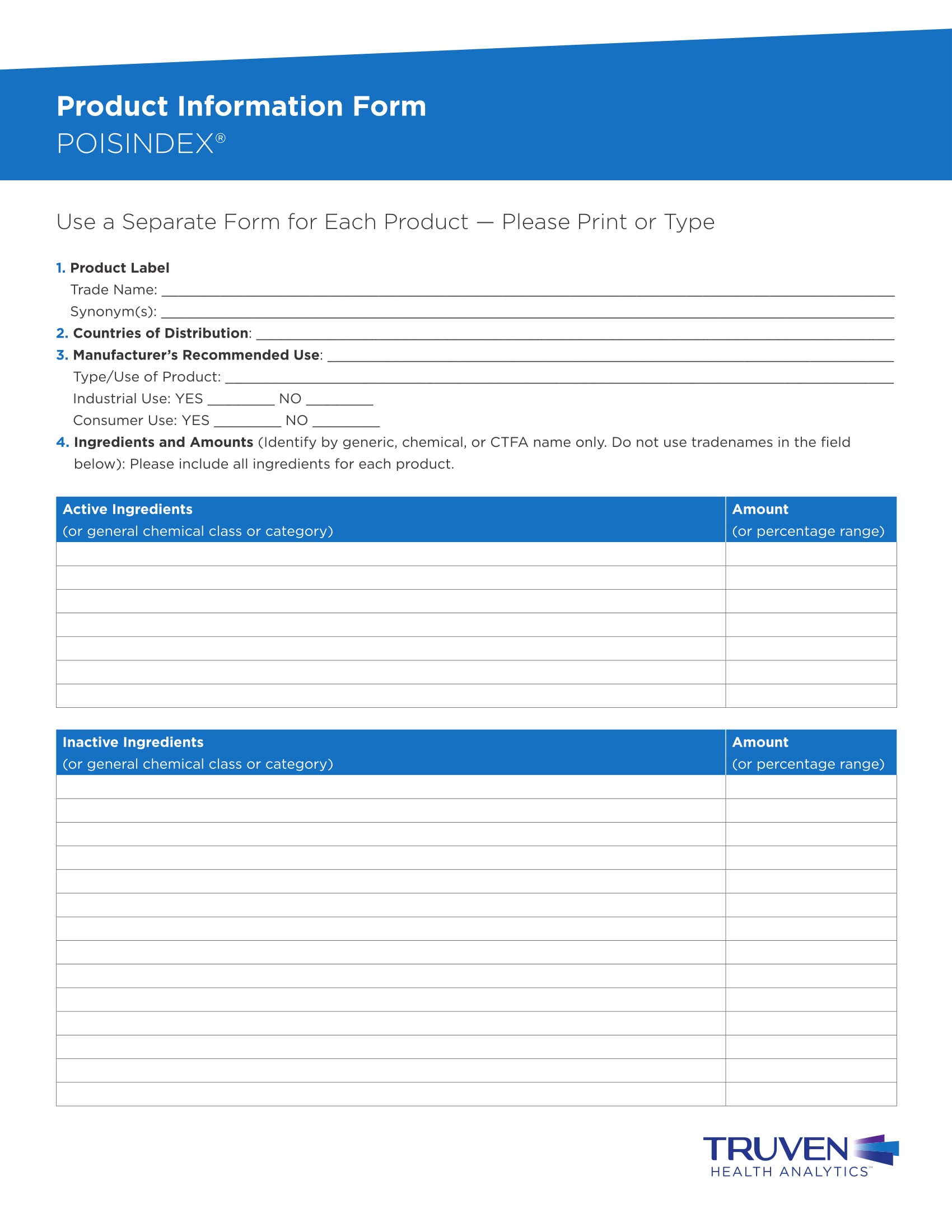
Free Product Information Sheet Templates For Google Sheets And
European medicines agency website (e.g. This guide is for applicants participating in the epi pilot. It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. The european medicines agency (ema) makes guidance and templates available to.
Ema Product Information Templates
It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. European medicines agency website (e.g. This guide is for applicants participating in the epi pilot. The european medicines agency's (ema) working group on quality review of documents (qrd).
FREE 14+ Product Information Forms in MS Word PDF Excel
This guide is for applicants participating in the epi pilot. European medicines agency website (e.g. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to.
Ema Product Information Templates prntbl.concejomunicipaldechinu.gov.co
This guide is for applicants participating in the epi pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for. European medicines agency website (e.g. It presents the electronic product information (epi) process that.
The European Medicines Agency's (Ema) Working Group On Quality Review Of Documents (Qrd) Develops, Reviews And Updates Templates For.
It presents the electronic product information (epi) process that will be used during the pilot. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with practical. This guide is for applicants participating in the epi pilot. European medicines agency website (e.g.